Basic scientific information
Integrating animal health surveillance and food safety: the example of Anisakis
Rev. sci. tech. Off. int. Epiz., 2013, 32 (2), 487-496
Integrating animal health surveillance and food safety: the example of Anisakis
E.Pozio
European Union Reference Laboratory for Parasites, Istituto Superiore di Sanità, viale Regina Elena 299, 00161 Rome, Italy
E-mail: edoardo.pozio@iss.it
Summary
Nematodes of the genera Anisakis and Pseudoterranova (family Anisakidae) are zoonotic parasites for which marine mammals (e.g. whales, dolphins, porpoises, seals, sea lions, walruses) act as final hosts, and crustaceans, cephalopods and fish as intermediate and/or paratenic hosts. In humans, the ingestion of Anisakidae larvae can result in infection with live larvae, an allergic reaction to Anisakidae allergens (even when dead larvae are ingested), or both. Worldwide, more than 2,000 infections are diagnosed in humans every year, yet most of the infections and allergic reactions are undiagnosed. A very high prevalence of anisakid larvae has been found in many commercially important species of fish, cephalopods and crustaceans. Preventive measures for anisakiosis focus on post-harvest handling.
Keywords
Anisakiosis – Anisakis – Cephalopod – Crustacean – Foodborne
Introduction
Nematode worms of the families Anisakidae and Raphidascarididae, which are commonly referred to as ‘anisakids’, are cosmopolitan parasites of marine mammals and fish-eating birds. The most important zoonotic species are those that belong to the genera Anisakis (A. simplex s.s. and A. pegreffii) and Pseudoterranova (P. decipiens) (34). Crustaceans serve as first intermediate hosts. Many species of cephalopods and saltwater fish (including anadromous fish) are second intermediate or paratenic hosts and are the source of infection for mammals, including humans, and birds. The prevalence of infection in fish and cephalopods varies greatly, depending on the host species and age, as well as on the fishing area. Humans become infected by consuming raw fish or cephalopods, which harbour anisakid larvae in the coelomic cavity, viscera or muscles. Humans can also develop allergic forms when they come into contact with the allergens in these worms, even if the fish is cooked. In the present review, the author attempts to provide a complete description of the two nematode genera that include the zoonotic species, and the infection caused by these parasites, including the epidemiology, prevalence, risk factors, diagnosis, treatment, and control measures.
The natural cycle
The eggs produced by the adult worms of Anisakis, which are found mainly in the stomachs of cetaceans (e.g. whales, dolphins and porpoises), and of Pseudoterranova, which are found mainly in the stomachs of pinnipeds (e.g. seals, sea lions and walruses), are excreted with faeces and hatch in the water (Fig. 1). The newly hatched larvae, which can survive in seawater for weeks, are eaten by a wide variety of crustaceans (e.g. copepods, amphipods, isopods, euphausiids and decapods) and molluscs (30, 44). The most important first intermediate hosts in the life cycle of Anisakis species are Euphausiids (krill), whereas copepods are the most important hosts in the life cycle of Pseudoterranova species (30). The prevalence of infection in these hosts is generally low (<1%), as is the larval burden (i.e. rarely more than one worm per host) (30). When infected crustaceans are eaten by fish and cephalopods, the larvae migrate to the coelomic cavity, particularly to the liver, gonads and intestine, where they become encapsulated (Fig. 1). When another fish or cephalopod ingests the infected fish, the new host acts as a paratenic host (i.e. the parasites do not develop further and remain at the L3 stage). Definitive hosts acquire the infection by eating infected fish, cephalopods or crustaceans. In the definitive host, the Anisakidae larvae rapidly grow to the L4 stage and then to adults (Fig. 1).
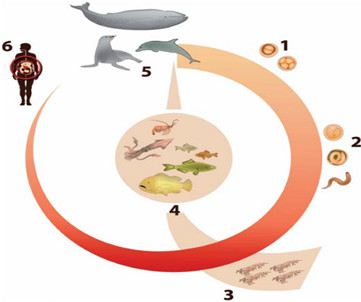
Natural cycle of zoonotic species of the genera Anisakis and Pseudoterranova
- Worm eggs are shed with faeces of marine mammals in seawater
- The embryo develops to the L1–L3 stage in the egg, after which the larva is released into seawater
- Euphausiids (krill) and copepods ingest the L1–L3 stages, which develop to the L3 stage or remain at the L3 stage
- The L3 stage is ingested by fish, crustaceans and cephalopods, which act as paratenic hosts
- When a marine mammal ingests fish, crustaceans and cephalopods, the L3 develops to L4 and then to the adult stage in the gut
- When humans accidentally ingest infected fish, crustaceans and cephalopods, one or more larvae can induce a clinical disease and/or an allergic reaction
Fig. 1 Natural cycle of zoonotic species of the genera Anisakis and Pseudoterranova
The eggs excreted with faeces by the definitive host release free-swimming larvae at either the L1 stage or L2 stage. When L1 stage larvae are eaten by crustaceans, they develop to the L2 stage; they are then eaten by fish and cephalopods, where they develop to the L3 stage (30, 44). According to other authors, the eggs release larvae already in the L3 stage (27). When a fish infected with L3 stage larvae is eaten by another fish, the larval fish-host cycle is repeated. This is important in terms of the epidemiology of infection and food safety, given that the repeated transfer of larvae among fish that belong to the natural food chain may result in the extensive accumulation of larvae, especially in large and older fish, which can harbour hundreds or even thousands of encapsulated larvae and thus increase the probability of infecting other fish and humans (44).
Primary fish hosts are predominantly planktivores, such as herring, haddock, blue whiting, and juvenile plaice, mackerel and cod, which acquire the parasite directly from crustacean hosts (1, 31). Secondary fish hosts are piscivores, such as blue shark, barracuda, monkfish and conger eel, which usually acquire the parasite from infected planktivorous fish (1, 31).
Taxonomy of nematodes of the genera Anisakis and Pseudoterranova
The current taxonomy of the two genera that belong to the Anisakidae family has been assessed in a recent review (34). In the Anisakis genus, two clades have been identified:
– Clade 1 (formerly known as Anisakis type 1), which includes A. simplex s.s., A. pegreffii and A. simplex C, which belong to the A. simplex complex, and A. typica, A. ziphidarum and Anisakis sp.
– Clade 2 (formerly known as Anisakis type 2), which includes A. physeteris, A. brevispiculata and A. paggiae.
In the Pseudoterranova genus, six species have been recognised:
– P. krabbei (formerly known as P. decipiens A)
– P. decipiens s.s. (formerly P. decipiens B)
– P. bulbosa (formerly P. decipiens C)
– P. azarasi (formerly P. decipiens D)
– P. decipiens E
– P. cattani.
Prevalence of infection and larval burden of Anisakidae in fish
A very large number of fish and cephalopod species act as hosts for Anisakis spp. (200 fish and 25 cephalopod species) and Pseudoterranova spp. (75 fish species in the North Atlantic alone); it is believed that most species of fish and cephalopods can potentially harbour these parasites (1, 32). According to some studies, there is seasonal variation in the prevalence of fish infection; however, this variation seems to be related to the weight and age of fish, with a higher prevalence and larval burden in larger and older fish (13). The prevalence of infection in fish and cephalopods depends on their specific feeding behaviour and biology and can range from lower than 1% to close to 100%.
In recent decades, the prevalence of infection among fish and cephalopods has increased, which is probably related to three factors:
– the increasing population size of the potential definitive hosts, due to the increasing public concern about the protection of marine mammals
– the practice of waste elimination from fishing vessels (i.e. throwing the waste into the sea) (1, 32)
– the increasing attention focused on these zoonotic parasites and their impact on public health.
From a public health point of view, the migration of larvae to the muscles in fish is epidemiologically important, in that the muscles are the part of the fish that is consumed by humans. In live fish, it is still unclear whether the migration of larvae is influenced by the Anisakidae species, or by the host species or age. When the fish dies, it is believed that larvae can migrate from the coelomic cavity to the muscles, depending on the environmental conditions to which the fish are subjected after they are caught. The longer the time between death and evisceration the higher the number of larvae in the muscles. Although the larvae can also reach distal muscles, most of them remain trapped in the bellyflaps of the fish (25).
Epidemiology of human infection (anisakiosis)
The larvae that can infect humans are those that belong to the species A. simplex, A. physeteris, A. pegreffii and P. decipiens; the most commonly implicated species are A. simplex, A. pegreffii and P. decipiens. There has also been a report of a case of infection with Contracaecum osculatum (43), though this case is questionable and the zoonotic role of this species needs to be investigated further.
In humans, the ingestion of Anisakidae larvae can result in infection with live larvae, an allergic reaction to Anisakidae allergens (even when dead larvae are ingested), or both. When humans ingest raw or undercooked fish infected with L3 larvae, the larvae penetrate the gastric or intestinal mucosa, resulting in gastric or intestinal pain and vomiting, with leucocytosis and in some cases eosinophilia (44).
Human infection is most frequently diagnosed in countries where it is common to eat raw and undercooked fish. Although the cooking or freezing of fish limits the risk of acquiring infection, it does not prevent allergic reactions to the antigens present in dead anisakids. In recent years, A. simplex has been identified as the aetiological agent of allergic reactions mediated by immunoglobulin (Ig)E antibodies (21). These allergic reactions range from rapid onset and potentially lethal anaphylactic reactions to chronic, debilitating conditions. In Spain, recently described allergic reactions included clinical symptoms that ranged from isolated angioedema/urticaria to lifethreatening anaphylactic shock. Contact dermatitis and occupational diseases have also been reported.
Economic impact of Anisakidae worms
A high prevalence of anisakid larvae has been found in commercially important species of fish, cephalopods and crustaceans. In fresh commercial salmon from the United States, the prevalence has been reported to exceed 75%. In herring from the English Channel, the prevalence of A. simplex larvae ranges from 78% to 97%. The effects of anisakids in terms of decreasing the commercial value of fish (47) and the impact on human health of infection and allergic reactions (7, 44) have resulted in these parasites becoming both an economic and a public health concern (19).
Risk factors for human infection
The prevalence of anisakiosis, like that of other fishborne parasitic zoonoses, is clearly related to traditions of consuming raw, lightly cooked or marinated fish, such as Japanese sushi and sashimi, Dutch salted or smoked herring, Scandinavian gravlax (dry, cured salmon), Spanish boquerones en vinagre (pickled anchovies), Hawaiian lomilomi salmon (raw salmon), Filipino kinilaw (chopped, marinated fish), and Latin American ceviche (raw fish seasoned with lemon juice) (12). In Italy and Spain, a high larval burden has been reported for anchovies, which are traditionally served raw with vinegar sauce without prior freezing, and sardines, which are eaten ungutted and charcoal-grilled (5).
Control measures
Preventive or control measures for anisakiosis focus on the post-harvest handling, storage, and preparation of fish (1). The immediate evisceration of fish may reduce the zoonotic potential of the parasite by preventing the migration of larvae to the muscles of the host fish, yet, given that the viscera are generally thrown into the sea and eaten by other fish, this could increase the prevalence of infection (32). Visual inspection of fillets is used to detect larvae embedded near the surface. To detect larvae embedded deep in the flesh, the most common method is candling (i.e. shining a bright light through the fillet); however, often as few as onethird of heavily infected fish are detected (30). Candling is effective in fillets of up to 2.5 cm in thickness, whereas for thicker fillets no larvae are detected.
The pressing method is widely used to systematically detect nematode larvae in the flesh of fish. This method utilises the fluorescence of frozen larvae, which appear as brightly fluorescent spots, and consists of visually inspecting pressed (1 mm to 2 mm thickness) deep-frozen fillets under ultraviolet (UV) light (24). The bags and their contents are then deep-frozen (≤–18°C) for at least 12 h prior to visual inspection under 366 nm UV light. The digestion method, which uses a pepsin/hydrochloric acid solution, frees live and dead larvae from muscles (23). The method recovers virtually all larvae; however, it is time consuming and thus only used for specific surveys rather than mass screening.
A real-time polymerase chain reaction method, combined with an optimised DNA extraction procedure, has recently been developed for the identification of A. simplex in seafood products (29). The method is highly specific and sensitive, with a detection limit of 40 ppm in 25 g of sample. However, because of its high cost, it is not suitable for the mass screening of industrially produced fish.
Anisakid larvae are resistant to salting, cold-smoking and marinating, and they do not appear to be killed by microwaving (39). Thus, for home consumption, fish should be cooked until the core temperature reaches 60°C or higher, for at least 10 min (39).
The United States Food and Drug Administration (22) recommends that fish intended for raw or semi-raw consumption should be frozen at –35°C or below for 15 h, or at –20°C or below for at least seven days. Marine fish used for aquaculture or farm feed should also be frozen adequately before use. However, the freezing of fish and cephalopods does not prevent the occurrence of allergic reactions.
Human infection
Of the approximately 20,000 cases of anisakiosis reported to date worldwide, over 90% are from Japan (where approximately 2,000 cases are diagnosed annually), with most of the remaining cases in Spain, the Netherlands, Italy and Germany (7). When ingested, anisakid larvae invade the stomach or the intestinal wall. Pseudoterranova decipiens seems to be more often associated with gastric anisakiosis, whereas A. simplex s.l. is more often associated with intestinal anisakiosis (15, 38). Most of the larvae remain in the gastric or intestinal submucosa and in the chronic stages cause the formation of a granuloma. Rarely, larvae may penetrate other sites (e.g. omentum, mesentery, lymph nodes, pancreas, ovaries, lungs and liver) (33).
Signs and symptoms of human infection
The clinical course of gastric anisakiosis is characterised by an abrupt onset of symptoms (e.g. intermittent epigastric pain, nausea and vomiting), usually within 6 h of ingestion of the larvae (Table I). Epigastric pain is often very severe and may not respond to analgesics (41). In persons with intestinal anisakiosis, symptoms usually begin five to seven days after ingestion of the larvae (38).

In recent years, anisakiosis has often been reported to cause a strong allergic reaction within 2 h to 6 h of ingesting larvae. The clinical symptoms range from isolated swelling to urticaria and life-threatening anaphylactic shock (4, 7, 8). Most cases of allergic reactions have been reported in Spain, though cases have also been reported in Egypt, France, Italy, Japan, the Republic of Korea, Portugal and South Africa (31). The Anisakis allergens that cause an allergic reaction appear to be highly resistant to heat and freezing (7). However, a priming infection with live parasites may be required to induce sensitisation (4, 9).
Diagnosis of human infection
Because the symptoms of anisakiosis are not pathognomonic, gastric anisakiosis is often misdiagnosed as peptic ulcer, stomach tumour or stomach polyps, and intestinal anisakiosis can be misdiagnosed as appendicitis or peritonitis (39). The clinical diagnosis is usually made by performing endoscopy, or by radiological or ultrasound examination. Various immunological assays have been used for indirect diagnosis. Interpretation of the serological tests may be difficult because the sera of individuals with anisakiosis cross-react with antigens from closely related nematode species (e.g. Ascaris and Toxocara species).
Treatment of human infection
For acute gastric anisakiosis, it is usually necessary to remove the worms using a fibre-optic endoscope, which leads to the immediate improvement of symptoms (37). For all of the other forms of anisakiosis, the choice of treatment depends on the specific complications (e.g. surgical removal of granuloma). For intestinal anisakiosis, conservative treatment with isotonic glucose solution is recommended (44). If this treatment fails, it is usually necessary to surgically remove the affected tissue. At present, for treating anisakiosis, albendazole and ivermectin have been shown to be effective (17, 36).
Inactivation of Anisakidae larvae in fishery products
The critical control points for prevention are:
– fishing in areas believed to have a low prevalence of infection among fish
– the application of physicochemical treatments to fishery products to ensure the killing of larvae
– the physical separation of contaminated fishery products during processing. Because A. simplex allergens are highly resistant to heat and freezing (20, 21), treatments that kill Anisakidae in fishery products may not protect consumers against allergic reactions. According to EC Regulation 853/2004 (18), fish should be frozen at a temperature of no higher than –20°C in all parts of the product for no less than 24 h in the following cases:
– if the product is to be consumed raw or almost raw
– if the product is from fish which should be undergoing a cold-smoking process (internal temperature < 60°C)
– if the product is a marinated and/or salted product and the processing is insufficient to destroy larvae.
The freezing treatment must be applied to either the raw or the finished product.
In the United States, the Food and Drug Administration (22) requires that all fish and shellfish intended to be consumed raw or semi-raw (e.g. marinated or partially cooked) be blast frozen to –35°C or below for 15 h, or be completely frozen to –20°C or below for seven days. The same freezing treatment is required in Canada. The Codex Alimentarius standard for salted Atlantic herring and salted sprats states that the viability of nematodes shall be examined after artificial digestion with magnetic stirring treatment (14). If live nematodes are detected, the product must not be placed on the market for human consumption unless it is frozen at –20°C for 24 h (in the core of the product).
Anisakidae larvae are sensitive to salt only under certain conditions. It has been estimated that 28 days of storage in brine with 6.3% salt and 3.7% acetic acid in the aqueous phase of the fish is the maximum survival time of the larvae in herring (26). Under industrial production conditions for dry salted herring, the total time needed to kill the parasites is 20 days (11).
Marinating is the process of soaking foods in a seasoned, often acidic, liquid with or without cooking; the active ingredients of the marinade can include vinegar, lemon juice, wine, soy sauce or brine. Early studies showed that A. simplex larvae are resistant to traditional conditions of marinating and can survive for 25 days in a mixture of salt and vinegar (28); depending on the salt concentration, the survival of larvae can reach 35 to 119 days (26). A marinade of vinegar (6% acetic acid) and 10% sodium chloride applied for 24 h to sardines, followed by the addition of sunflower seed oil and refrigeration for 13 days, inactivated A. simplex larvae (6) (Table II). Factors that influence the effectiveness of freezing for inactivating anisakid larvae include the temperature, the time needed to reach the final temperature in the core fish tissues, the duration of freezing, and the fat content of the fish (48).
Studies have shown that a core temperature of 60°C for 1 min is sufficient to kill any larvae present in a fishery product (10). However, reaching such a core temperature depends on the thickness and composition of the product. Hydrostatic pressure kills A. simplex larvae (200 MPa for 10 min at 0°C to 15°C, or 140 MPa for 60 min at 0°C to 15°C) (35). Larvae of A. simplex can be killed by an irradiation dose higher than 6 to 10 kGy.
Hot smoking at 70°C to 80°C for 3 h to 8 h is sufficient to kill A. simplex larvae. By contrast, cold-smoking (< 38°C) is not sufficient (45) and these products must thus undergo an initial inactivation treatment. Freezing raw products prior to smoking remains the most effective way of ensuring that viable parasites are killed in cold-smoked products.

Conclusions
The epidemiology and clinical patterns in humans of infection with nematodes of the family Anisakidae are complex. Consequently, to deal with the Anisakis problem, there is a need for strong cooperation between the authorities responsible for animal health and public health/ food safety. The main issues are: the information provided to the fishing industry on the risk derived from the practice of waste elimination from fishing vessels (i.e. throwing the waste into the sea); the methods used to check for the presence of the nematode larvae; and the methods used to inactivate these parasites in fishery products. Furthermore, the education and information provided to consumers, physicians and people working in the catering industry should be improved.
La surveillance intégrée de la santé animale et de la sécurité
sanitaire des aliments : l’exemple d’Anisakis
- Pozio
Résumé
Les nématodes appartenant aux genres Anisakis et Pseudoterranova (famille des Anisakidés) sont des parasites zoonotiques ayant pour hôtes définitifs des espèces de mammifères marins (par ex. les baleines, les dauphins, les marsouins, les phoques, les otaries et les morses) et pour hôtes intermédiaires et/ou paraténiques les crustacés, les céphalopodes et les poissons. Chez l’homme, l’ingestion de larves d’Anisakidés peut provoquer une infestation par des larves vivantes et/ou une réaction allergique aux allergènes contenus dans celles-ci (même en cas d’ingestion de larves mortes). À l’échelle mondiale, plus de 2 000 cas humains d’anisakiase sont diagnostiqués chaque année ; néanmoins, la plupart des cas d’infestation ou de réaction allergique ne font pas l’objet d’un diagnostic. Une prévalence extrêmement élevée de larves d’Anisakidés a été retrouvée dans la chair de nombreuses espèces de poissons, de céphalopodes et de crustacés très présentes dans le commerce. Les mesures de prévention de l’anisakiase sont axées sur une bonne gestion post-collecte.
Mots-clés
Anisakiase – Anisakis – Céphalopode – Crustacé – Maladie d’origine alimentaire –
Mammifère marin – Nématode – Poisson marin – Pseudoterranova – Zoonose.
Integración de vigilancia zoosanitaria e higiene de los alimentos: el ejemplo de Anisakis
- Pozio
Resumen
Los nematodos de los géneros Anisakis y Pseudoterranova (familia Anisakidae) son parásitos zoonóticos que tienen como hospedadores finales a mamíferos marinos (ballenas, delfines, marsopas, focas, leones marinos, morsas, etc.) y como hospedadores intermedios y/o paraténicos a crustáceos, cefalópodos y peces. En el ser humano, la ingestión de larvas de anisákidos puede causar bien la infección por larvas vivas o bien una reacción alérgica a ciertos alergenos presentes en el parásito (aun cuando la persona haya ingerido larvas muertas), o a veces ambas cosas. Cada año se diagnostican en el mundo más de 2.000 casos de infestación humana, aunque la mayoría de las infestaciones y reacciones alérgicas no son diagnosticadas. En numerosas especies de peces, cefalópodos y crustáceos comercialmente importantes se ha hallado una prevalencia muy elevada de larvas de anisákidos. Las medidas de prevención de la anisakiosis giran esencialmente en torno a la manipulación de los animales muertos.
Palabras clave
Anisakiosis – Anisakis – Cefalópodo – Crustáceo – Mamífero marino – Nematodo – Pez marino – Pseudoterranova – Transmisión por vía alimentaria – Zoonosis.
References
- Abollo E., Gestal C. & Pascual S. (2001). – Anisakis infestation in marine fish and cephalopods from Galicia waters: an update perspective. Parasitol. Res., 87, 492–499.
- Adams A.M., Ton M.N., Wekell M.M., MacKenzie A.P. & Dong F.M. (2005). – Survival of Anisakis simplex in arrowtooth flounder (Atheresthes stomia) during frozen storage. J. Food Protec., 68, 1441–1446.
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) (2007). – Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición sobre medidas para reducir el riesgo asociado a la presencia de Anisakis. Available at: www.aesan.msc.es/AESAN/web/ cadena_alimentaria/detalle/anisakis.shtml (accessed on 18 April 2013).
- Alonso A., Moreno-Ancillo A., Daschner A. & López- Serrano M.E. (1999). – Dietary assessment in five cases of allergic reactions due to gastroallergic anisakiasis. Allergy, 54, 517–520.
- Alonso-Gómez A., Moreno-Ancillo A., López-Serrano M.E., Suarez-de-Parga J.M., Daschner A., Caballero M.T., Barranco P. & Cabañas R. (2004). – Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol. Res., 93, 378–384.
- Arcangeli G., Galuppi A., Bicchieri M.G.R. & Presicce M. (1996). – Prove sperimentali sulla vitalità di larve del genere Anisakis in semiconserve ittiche. Industria Conserve, 71, 502–507.
- Audicana M.T., Ansotegui I.J., de Corres L.E. & Kennedy M.W. (2002). – Anisakis simplex: dangerous – dead and alive? Trends Parasitol., 18, 20–25.
- Audicana M.T. & Kennedy M.W. (2008). – Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev., 21, 360–379.
- Baeza M.L., Rodríguez A., Matheu V., Rubio M., Tornero P., de Barrio M., Herrero T., Santaolalla M. & Zubeldia J.M. (2004). – Characterization of allergens secreted by Anisakis simplex parasite: clinical relevance in comparison with somatic allergens. Clin. experim. Allergy, 34, 296–302.
- Bier J.W. (1976). – Experimental anisakiasis: cultivation and temperature tolerance determinations. J. Milk Food Technol., 39, 132–137.
- Centre d’étude et de valorisation des produits de la mer (CEVPM) (2005). – Etude des conditions de destruction des larves d’Anisakis simplex dans le hareng salé au sel sec destiné à la fabrication de harengs saurs traditionnels. Available at: www.bibliomer.com/consult.php?ID=2007-4062 (accessed on 18 April 2013).
- Chai J.Y., Murrell K.D. & Lymbery A.J. (2005). – Fish-borne zoonoses: status and issues. Int. J. Parasitol., 35, 1233–1254.
- Chou Y.Y., Wang C.S., Chen H.G., Chen H.Y., Chen S.N. & Shih H.H. (2011). – Parasitism between Anisakis simplex (Nematoda: Anisakidae) third-stage larvae and the spotted mackerel Scomber australasicus with regard to the application of stock identification. Vet. Parasitol., 177, 324–331.
- Codex Alimentarius Commission (2004). – Standard for salted Atlantic herring and salted sprat. CODEX STAN 244-2004. Available at: www.codexalimentarius.org/standards/list-ofstandards/ en/?no_cache=1 (accessed on 18 April 2013)
- Couture E., Measures L., Gagnon J. & Desbiens E. (2003). – Human intestinal anisakiosis due to consumption of raw salmon. Am. J. Surg. Pathol., 27, 1167–1172.
- Deardorff T.L. & Throm R. (1988). – Commercial blastfreezing of third-stage Anisakis simplex larvae encapsulated in salmon and rockfish. J. Parasitol., 74, 600–603.
- Dziekonska-Rynko J., Rokicki J. & Jablonowski Z. (2002). – Effects of ivermectin and albendazole against Anisakis simplex in vitro and in guinea pigs. J. Parasitol., 88, 395–398.
- European Commission (EC) (2004). – Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules on the hygiene of foodstuffs. Off. J. Eur. Union, L139, 55–95.
- European Food Safety Authority (EFSA) (2010). – Scientific opinion on risk assessment of parasites in fishery products. EFSA J., 8, 1543–1634.
- Falcão H., Lunet N., Neves E., Iglésias I. & Barros H. (2008). – Anisakis simplex as a risk factor for relapsing acute urticaria: a case-control study. J. Epidemiol. community Hlth, 62, 634–637.
- Fernández de Corres L., Audicana M., Del Pozo M.D., Muñoz D., Fernández E., Navarro J.A., García M. & Diez J. (1996). – Anisakis simplex induces not only anisakiasis: report on 28 cases of allergy caused by this nematode. J. invest. Allergol. clin. Immunol., 6, 315–319.
- Food and Drug Administration (FDA) (USA) (2011). – Chapter 5: Parasites. In Fish and fishery products: hazards and controls guidance. Available at: www.fda.gov/downloads/ Food/GuidanceRegulation/UCM252393.pdf (accessed on 18 April 2013).
- Jackson G.J., Bier J.W., Payne W.L. & McClure F.D. (1981). – Recovery of parasitic nematodes from fish by digestion or elution. Appl. environ. Microbiol., 41, 912–914.
- Karl H. & Leinemann M. (1993). – A fast and quantitative detection method for nematodes in fish fillets and fishery products. Arch. Lebensmittelhyg., 44, 105–128.
- Karl H., Meyer C., Banneke S., Sipos G., Bartelt E., Lagrange F., Jark U. & Feldhusen F. (2002). – The abundance of nematode larvae Anisakis sp. in the flesh of fishes and possible postmortem migration. Archiv Lebensmittelhygiene, 53, 119–111.
- Karl H., Roepstorff A., Huss H.H. & Bloemsma B. (1995). – Survival of Anisakis larvae in marinated herring fillets. Int. J. Food Sci. Technol., 29, 661–670.
- Køie M., Berland B. & Burt M.D.B. (1995). – Development to third-stage larvae occurs in the eggs of Anisakis simplex and Pseudoterranova decipiens (Nematoda, Ascaridoidea, Anisakidae). National Research Council of Canada, Ottawa.
- Kuipers F.C., Rodenburg W., Wielinga W.J. & Roskam R.T. (1960). – Eosinophilic phlegmon of the alimentary canal caused by a worm. Lancet, 2, 1171–1173.
- López I. & Pardo M.A. (2010). – Evaluation of a real-time polymerase chain reaction (PCR) assay for detection of Anisakis simplex parasite as a food-borne allergen source in seafood products. J. agric. Food Chem., 58, 1469–1477.
- McClelland G. (2002). – The trouble with sealworms (Pseudoterranova decipiens species complex, Nematoda): a review. Parasitology, 124, S183–S203.
- McClelland G. & Martell D.J. (2001). – Surveys of larval sealworm (Pseudoterranova decipiens) infection in various fish species sampled from Nova Scotian waters between 1988 and 1996, with an assessment of examination procedures. In Sealworms in the North Atlantic: ecology and population dynamics (G. Desportes & G. McClelland, eds). North Atlantic Marine Mammal Commission, Tromsø, Norway, 57–76.
- McClelland G., Misra R.K. & Martell D.J. (1990). – Larval anisakine nematodes in various fish species from Sable Island Bank and vicinity. Can. Bull. Fish. aquat. Sci., 222, 83–118.
- Matsuoka H., Nakama T., Kisanuki H., Uno H., Tachibana N., Tsubouchi H., Horii Y. & Nawa Y. (1994). – A case report of serologically diagnosed pulmonary anisakiasis with pleural effusion and multiple lesions. Am. J. trop. Med. Hyg., 51, 819–822.
- Mattiucci S. & Nascetti G. (2008). – Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv. Parasitol., 66, 47–148.
- Molina-García A.D. & Sanz P.D. (2002). – Anisakis simplex larva killed by high-hydrostatic pressure processing. J. Food Protec., 65, 383–388.
- Moore D.A., Girdwood R.W. & Chiodini P.L. (2002). – Treatment of anisakiasis with albendazole. Lancet, 360, 54.
- Noh J.H., Kim B., Kim S.M., Ock M., Park M.I. & Goo J.Y. (2003). – A case of acute gastric anisakiasis provoking severe clinical problems by multiple infection. Korean J. Parasitol., 41, 97–100.
- Oshima T. (1987). – Anisakiasis: is the sushi bar guilty? Parasitol. Today, 3, 44–48.
- Sakanari J.A. & McKerrow J.H. (1989). – Anisakiasis. Clin. Microbiol. Rev., 2, 278–284.
- Sánchez-Monsalvez I., de Armas-Serra C., Martínez J., Dorado M., Sánchez A. & Rodríguez-Caabeiro F. (2005). – A new procedure for marinating fresh anchovies and ensuring the rapid destruction of Anisakis larvae. J. Food Protec., 68, 1066–1072.
- Sato I. (1992). – Clinical study of gastric anisakiasis. Akita J. Med., 19, 503–510.
